MA DPH Updated Comprehensive PPE Guidance
The Massachusetts Department of Public Health is releasing updated Comprehensive Personal Protective Equipment (PPE) Guidance. This guidance further updates the PPE standards for health care personnel. As part of this updated guidance, healthcare providers may implement policies to require HCP to use employer-issued PPE, and to preclude staff from using their own PPE absent appropriate safety and quality controls which may include but are not limited to fit-testing.
Memorandum
TO: Health Care Facility Chief Executive Officers and Administrators
Occupational Health Program Leaders
Emergency Medical Service Directors
FROM: Elizabeth Daake Kelley, MPH, MBA, Director
Bureau of Health Care Safety and Quality
SUBJECT: Comprehensive Personal Protective Equipment (PPE) Guidance
DATE: January 21, 2022
The Massachusetts Department of Public Health (DPH) continues to work with state, federal and local partners on the outbreak of Coronavirus Disease 2019 (COVID-19), caused by the virus SARS-CoV-2, and we continue to appreciate the essential role you have in responding to this evolving situation.
DPH has developed this comprehensive guidance, based upon the Centers for Disease Control and Prevention (CDC) recommendations, to clarify the PPE that health care personnel (HCP) use in clinical care areas and in other non-clinical areas in health care facilities. HCP refers to all paid and unpaid persons serving in healthcare settings and emergency medical services (EMS) who have the potential for direct or indirect exposure to patients or infectious materials including body substances; contaminated medical supplies, devices, and equipment; contaminated environmental surfaces; or contaminated air.[1] Healthcare settings are not limited to health care facilities but may include community settings where home health workers or EMS personnel are providing care to patients. This guidance further updates the PPE standards that HCP must follow. Healthcare providers may implement policies to require HCP to use employer-issued PPE, and to preclude staff from using their own PPE absent appropriate safety and quality controls which may include but are not limited to fit-testing.
These changes may be implemented immediately.
Universal Use of Facemasks
DPH has adopted a universal facemask use policy for all HCP. All HCP should don a facemask upon entry to the healthcare facility premises or care area (which includes ambulances); HCP working in community settings should don a facemask upon entry to the home or immediate area where they will be providing assessment and care. Facemasks are defined as surgical or procedure masks worn to protect the mouth/nose against infectious materials and have been shown to be highly effective at preventing transmission of COVID-19 when both individuals are masked. This policy will have two presumed benefits. The first benefit is to prevent pre-symptomatic spread of COVID-19 from HCP to patients, visitors and colleagues by reducing the transmission of droplets. The second benefit is to protect HCP by reducing transmission from their surroundings, including from other staff, visitors and patients who may be in a pre-symptomatic stage.
Extended use of facemasks is the practice of wearing the same facemask for repeated encounters with several different patients without removing the facemask between patient encounters. Due to improvement in the health care supply chain of facemasks, DPH is modifying earlier guidance and supports face mask use as follows:
As PPE to protect their nose and mouth from exposure to splashes, sprays, splatter, and respiratory secretions (e.g., for patients on Droplet Precautions). When used for this purpose, facemasks should be removed and discarded after each patient encounter.
As source control to cover one’s mouth and nose to prevent spread of respiratory secretions when they are talking, sneezing, or coughing. When used for this purpose, facemasks may be used for multiple patient encounters under the following conditions:
· The facemask should be removed and discarded if soiled, damaged, or hard to breathe through.
· HCP must take care not to touch their facemask. If they touch or adjust their facemask they must immediately perform hand hygiene.
· HCP should leave the clinical care area if they need to remove the facemask. (i.e., outside of the patient room)
· Facemasks should not be stored or put down on a surface; when removed, facemasks should be discarded and HCP should don a new facemask.
· If HCP remove their facemask to eat, drink or during a break they should perform hand hygiene with soap and water or an alcohol-based hand rub before and after touching their mask.
Homemade and cloth facemasks are not considered PPE and are not appropriate for use in the healthcare setting or by HCP.
As part of universal source control, if tolerated, patients/residents should wear a facemask issued by their provider when they leave their room or when staff are providing care to them.
PPE for patients with suspected or confirmed COVID-19, or confirmed exposures to COVID-19
DPH recommends that a fit-tested N95 filtering facepiece respirator or alternative, eye protection, isolation gown and gloves be used when caring for patients with suspected or confirmed COVID-19 or confirmed exposure to COVID-19.
Respirators:
Proper use of respiratory protection by HCP requires a comprehensive program (including medical clearance, training, and fit testing) that complies with OSHA’s Respiratory Protection Standard.
Facilities should eliminate the practice of reuse of N95 respirators with the exception of conditions outlined in Other Considerations. N95 respirators should always be discarded after doffing, such as when leaving a patient room, during a break or when eating or drinking. Respirators contaminated with blood, respiratory or nasal secretions, or other bodily fluids must be discarded immediately.
If reusable N95 respirator alternatives such as elastomeric respirators are used each facility must ensure appropriate cleaning and disinfection between uses and filter exchange according to manufacturer’s instructions. When used as source control, reusable N95 respirator alternatives may be worn between patients seen sequentially without cleaning and disinfection. If worn when seeing patients on transmission-based precautions, extended use may be performed if not contaminated.
Eye Protection:
At this time, when the risk of community transmission of COVID-19 in Massachusetts continues to be increased, HCP should wear eye protection for all patient encounters.
Disposable eye protection should be removed and discarded when it is removed for any reason; it should not be reused. Reusable eye protection should be cleaned and disinfected when visibly soiled and after removal/doffing of eye protection. Eye protection may be used for multiple patient encounters under the following conditions:
Eye protection should be removed and reprocessed if it becomes visibly soiled or difficult to see through.
Eye protection should be discarded if it becomes damaged (e.g., face shield can no longer fasten securely to the provider, if visibility is obscured and reprocessing does not restore visibility).
If reusable goggles or face shields are used each facility must ensure appropriate cleaning and disinfection between uses according to manufacturer’s instructions.
After cleaning and disinfection, reusable eye protection should be stored in a designated location.
HCP should not touch their eye protection while being worn. If they touch or adjust their eye protection hand hygiene must be performed.
HCP should leave the clinical care area if they need to remove their eye protection using recommended protocols for removing, cleaning, and disinfecting, and reprocessing.
Isolation Gowns:
Nonsterile, disposable patient isolation gowns, which are used for routine patient care in healthcare settings, are appropriate for use by HCP when caring for patients with suspected or confirmed COVID-19 or confirmed exposure. HCP may also use reusable (i.e., washable) gowns made of polyester or polyester-cotton fabrics; they can be safely laundered according to routine procedures and reused. Reusable gowns should be replaced when thin or ripped, and per the manufacturer’s instructions. Gowns should be disposed of or laundered after each patient encounter.
Any gown that becomes visibly soiled during patient care should be disposed of or laundered, as appropriate.
Gloves:
HCP should perform hand hygiene prior to donning and after doffing gloves.
Other Considerations:
For performing aerosol generating procedures, such as nebulizer treatments or intubations, HCP should don a fit-tested N95 filtering facepiece respirator or acceptable alternate product except in the following circumstances when Standard Precautions may be used:
The patient has recovered from COVID-19 within the previous 90 days;
The patient is asymptomatic, and a COVID-19 test obtained within the past three days is negative.
Health care organizations and providers that are caring for high numbers of patients with suspected or confirmed COVID-19, or confirmed exposures to COVID-19 during high rates of community transmission may choose to adopt any of the following principles when caring for patients in the same cohort (i.e. all confirmed COVID-19 cases):
Utilize the same N95 respirator or other acceptable alternate product between multiple patient encounters provided that the N95 respirator or acceptable alternative is always discarded after doffing, during a break, when eating or drinking or when contaminated with blood, respiratory or nasal secretions, or other bodily fluids.
Reuse the same N95 respirator or acceptable alternate product over the course of an entire shift; N95 respirators or acceptable alternative must be discarded if non-intact or contaminated with blood, respiratory or nasal secretions, or other bodily fluids.
Utilize the same eye protection between multiple patient encounters provided that the eye protection is clean and disinfected after doffing, or when contaminated with blood, respiratory or nasal secretions, or other bodily fluids.
Resources:
Health care organizations and providers that require additional PPE in order to meet the use standards described in this guidance and are not able to obtain through their usual supply chain resources may request support from DPH as a bridge until health care organizations increase their ordering and receipt of gloves, eye protection, facemasks, gowns and N95 respirators. DPH will review requests and provide additional PPE on a monthly basis for the months of January, February and March as a bridge supply for health care organizations and providers that have an immediate and insufficient supply for HCP caring for individuals with suspected or confirmed COVID-19 or exposures to COVID-19. Every health care organization must immediately adjust their supply order to ensure that going forward they have sufficient supplies to meet this guidance. A health care organization or provider who has insufficient supply should fill out and download the PPE request form and submit it via email to Covid19.resource.request@mass.gov.
The form may be found on DPH’s website:
https://www.mass.gov/info-details/personal-protective-equipment-ppe-during-covid-19. Please visit
DPH’s website that provides up-to-date information and guidance documents on COVID-19 for healthcare providers and organizations in Massachusetts:
Isolation and Quarantine Guidance for Health Care Personnel
Dear Colleagues,
The Massachusetts Department of Public Health is sharing updated isolation guidance for healthcare personnel. The updated isolation guidance applies to healthcare settings as defined by the CDC. The updated guidance reduces the amount of time healthcare personnel must isolate due to testing positive for COVID-19 under a set of conditions as described in the updated guidance. Please see the attached document or the below link for details: https://www.mass.gov/doc/isolation-and-quarantine-guidance-for-health-care-personnel/download
Thank you for your continued care of your patients, staff and communities during this unprecedented time.
The Department of Public Health
Updated Comprehensive PPE Guidance
Dear Colleagues,
The Massachusetts Department of Public Health (DPH) is sharing updated Comprehensive Personal Protective Equipment (PPE) guidance. DPH is updating this guidance to:
Create greater flexibility to allow extended use of eye protection to align with CDC PPE use guidance issued at the end of September. DPH has clarified that reusable eye protection does not need to be cleaned in-between each patient encounter but as directed by the manufacturer and any time it is removed.
Update aerosol-generating procedure precautions based upon continued high levels of community spread. Providers must don N95 respirators or alternatives to perform these procedures unless there is a COVID-19 test within the past three days or the patient has recovered from COVID-19 within the past three months.
Clarify that this guidance applies to EMS personnel and home health workers in community settings.
Thank you for your ongoing dedication to your staff and patients throughout the COVID-19 pandemic.
Thank you,
The Massachusetts Department of Public Health
Commissioner's COVID-19 Orders Update
Updated information about the Commissioner’s Orders related to COVID-19 can now be found at www.mass.gov/info-details/covid-19-public-health-emergency
The Commonwealth’s web team is still working through updating the OEMS website, so updates may take time to show up there.
Rescinding of COVID-19 Advisories and Updates to Administrative Requirements
In keeping with the current guidance from the Governor, in response to the current status of the COVID-19 pandemic and the termination of the State of Emergency, the Department has rescinded the documents listed below, effective June 9, 2021:
· Advisory 20-10-01: COVID-19 workplace safety standards for ambulance services
· Advisory 20-05-01: In-person EMS courses and psychomotor exams during COVID-19
· Memorandum on modification of psychomotor exams during COVID-19
· Emergency Protocol 1.2: Patient at Risk of COVID-19
· Emergency Protocol 1.3: Emergency Non-Transport of COVID-19 Patients
· Advisory 20-03-03: Extension of First Responder Training Expirations
In addition, OEMS has updated the following Administrative Requirements (ARs), effective June 9, 2021, as attached:
· AR 2-212, EMT Continuing Education Standards
· AR 2-248, Deadlines for Renewal of Certification, and Reinstatement Procedures for Massachusetts Certified EMT-Basics
· AR 2-353, Deadlines for Renewal of Certification, and Reinstatement Procedures for Massachusetts Certified Advanced EMTs and Paramedics, to remove COVID-19 related provision.
Information related to Commissioner’s Orders and waivers issued pursuant to such Orders should be released in the coming days.
This is the COMPLETE AR 2-353, Deadlines for Renewal of Certification, and Reinstatement Procedures for Massachusetts Certified Advanced EMTs and Paramedics, to remove COVID-19 related provision.
Comprehensive Personal Protective Equipment (PPE) Guidance
Recently, the Centers for Disease Control and Prevention (CDC) updated its recommendations for personal protective equipment (PPE). Based upon updated recommendations shared by the CDC, the attached updated guidance document from the Massachusetts Department of Public Health (DPH) updates PPE recommendations for health care providers to reflect a greater availability of PPE and return to conventional uses of PPE, including N95 respirators. Healthcare providers should expect to return to conventional facemask and respirator use, meaning discarding as mask after each patient encounter, no later than July 1, 2021.
COVID-19 Vaccine Clinics for First Responders Updated BOH FAQs (UPDATED April 7, 2021)
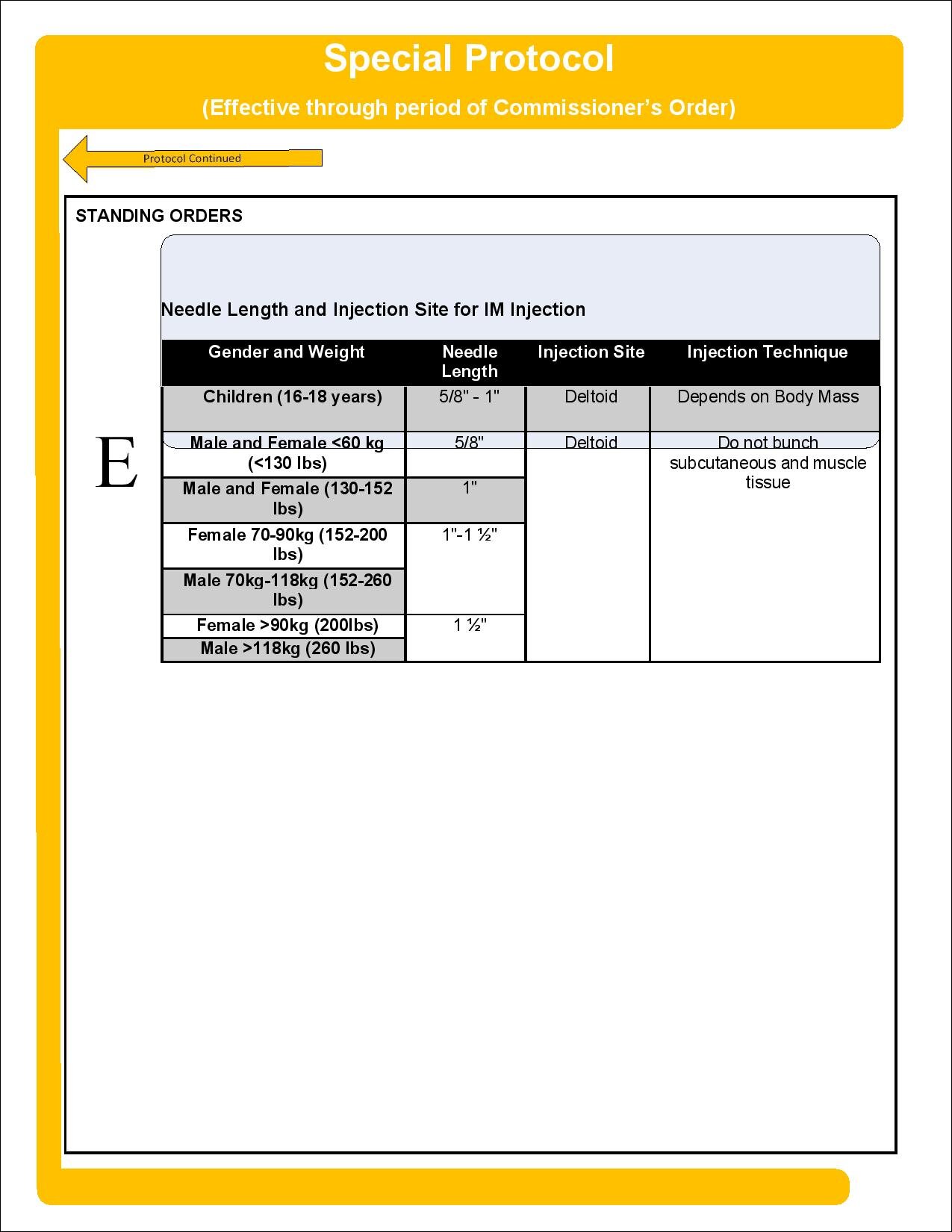
Update, April 7, 2021: The Department of Public Health’s Office of Emergency Medical Services (Department) has issued an updated Special Protocol for EMT-Basic COVID-19 Vaccine Administration. See attached. The sole update is to allow EMT-Basics, only when they are administering COVID-19 vaccine to an individual in their home, to receive their supervision and oversight from a physician, nurse or paramedic who is immediately available by phone or video. Otherwise, as before, all EMT-Basics vaccinating under the Commissioner’s Order must be working with at least one physician, nurse, or paramedic immediately available at the treatment site as the supervisor of dosing, procedure, and technique.
For any questions with regard to the temporary authorization for EMS personnel to administer COVID-19 vaccine, please contact renee.atherton@mass.gov.
This guidance answers commonly asked questions we have received from Local Boards of Health to support COVID-19 vaccine clinics for First Responders. Topics covered in this guidance include:
Vaccine providers can also refer to www.mass.gov/CovidVaccineProviders for additional information, including detailed Guidance on COVID-19 Vaccine Management and Administration for Healthcare Providers and Organizations and frequently asked questions from vaccine providers.
Timing and Populations to be Vaccinated
When will we be expected to stand up the first responder clinics?
Based upon current expectations of vaccine availability, you and your partners should be prepared to start scheduling of appointments during the week of January 4, 2021 and stand up your initial clinics during the week of January 11, 2021. Vaccination may not begin before January 11, 2021.
Who will be eligible to receive the COVID-19 vaccine at these clinics?
Emergency Medical Services (EMS), police, and fire personnel are eligible for vaccinations in the first responder clinics during Phase 1. This includes all interfacility transport workers, MedFlight staff, college/university campus police, and 911 Dispatch employees. Visit When can I get the COVID-19 vaccine? | Mass.gov often as the priority groups are updated frequently. These clinics will not be open to family or household members who are not currently employed as first responders. Please note that the vaccination needs of State Police will be addressed separately and are not included in these locally organized clinics.
Individuals seeking vaccination are required to provide documentation of eligibility, as described at COVID-19 Vaccine Locations for First Responders | Mass.gov. This page also includes a map of COVID-19 vaccine locations with contact details and sign-up information for First Responders.
May we vaccinate the vaccinators and administrative staff who will support clinic operations?
Yes, clinical staff who will be administering vaccine and support staff who will be patient-facing may be vaccinated. Staff who are assigned to positions in which they will not have direct contact with individuals receiving vaccine are not eligible. Please note that after patient-facing staff receive the initial dose of vaccine, they must continue to use appropriate personal protective equipment (PPE) at all times and practice hand hygiene and other health precautions.
If first responders choose not to get vaccinated at these clinics, will vaccine be available when they are ready?
Individuals in a priority group remain eligible during their phase of COVID-19 vaccination and any time thereafter. First responders are urged to access COVID-19 vaccine as soon as they are eligible. First responders will continue to have access to the vaccine through other sites, such as mass vaccination sites.
Will private ambulance services be vaccinating their own employees?
There may be some private ambulance services that will request vaccine directly for their employees. However, this is not the case for all services and any community that has arranged to vaccinate their private ambulance services should expect to continue with that arrangement.
Are we allowed to vaccinate other COVID-facing healthcare workers at these clinics? (New 1/13/21)
Yes, LBOH clinics are encouraged to vaccinate COVID-facing healthcare workers if they have the capacity. COVID-facing healthcare workers are required to provide documentation of eligibility, as described at COVID-19 vaccine locations for individuals currently eligible to be vaccinated | Mass.gov and should verify they are eligible for vaccine as a COVID-facing health care worker.
To ensure enough vaccine allocation for your site, sites will need to complete a weekly MCVP survey to ensure that DPH understands providers’ vaccine needs, the phase they are currently vaccinating in, as well as other information. The survey is also an option for the provider to request additional doses as part of the allocation process. If you have not received the weekly survey, please email DPH-Vaccine-Management@massmail.state.ma.us.
Administrative Considerations
What requirements must the local board of health meet in order to offer a COVID-19 vaccination program for first responders?
All organizations or providers receiving COVID-19 vaccine must execute the Massachusetts COVID-19 Vaccine Program (MCVP) Agreement. Among other things, this Agreement obligates providers to administer COVID-19 vaccine in accordance with the terms of the United States Food and Drug Administration (FDA) Emergency Use Authorization (EUA) applicable to the vaccine that will be administered. The MCVP Agreement is emailed as a link to contacts associated with the Massachusetts Immunization Information System (MIIS) and State Vaccine Program.
Sites enrolling in the MCVP must already be registered with the MIIS. Pursuant to G.L. c. 111 s. 24M and 105 CMR 222, licensed healthcare providers who administer immunizations are required to report certain information to the Department’s MIIS. COVID-19 vaccine providers must meet this reporting requirement by registering with the MIIS, which will include executing the MIIS Site and User Agreements.
You must have the capacity to fully organize and staff the clinics to meet the vaccination needs of at least 200 first responders to qualify. This includes local capacity to fully organize and staff the clinics, to safely store vaccine, and to bill insurance for administration-related costs if other local financial resources are not available. At this time, no funding for administration related costs is available through the federal government or from the state. The Commonwealth will allocate COVID-19 vaccine and selected ancillary supplies, including syringes and needles, to approved local health departments, subject to available supply, but each location must be able to provide its own refrigeration/freezer capacity, PPE, clinical and non-clinical staffing, and any other resources needed to support clinic operations.
You must have the ability to schedule vaccination appointments and ensure that individuals will receive their second dose of vaccine within the prescribed time frame. If you have a system in place to schedule appointments, you may continue to use that, or you may use PrepMod, a web-based system offered through DPH.
What is PrepMod? (Updated 1/6/21)
PrepMod is an online, paperless system that you can use at your first responder vaccination clinic to schedule, screen, bill, and report to the MIIS. The system provides companion technologies that automate registration, planning, implementation, evaluation, recording, and reporting for mass vaccination and preparedness efforts. Use of PrepMod is not required, but there is a federal requirement that all COVID-19 vaccine data be reported to the MIIS within 72 hours, and PrepMod will facilitate that process. If you enter all necessary information into PrepMod, it will be sent automatically to the MIIS so there is no need to enter the information again to satisfy required MIIS reporting. If you have questions about PrepMod, please contact Prepmod.help@mass.gov.
Is there a cost to providers to receive and administer the vaccine?
There is no cost for the vaccine or ancillary kits. The U.S. Centers for Medicare and Medicaid Services (CMS) has approved reimbursement for the administration of the vaccine. While vaccine providers may not bill for the COVID-19 vaccine itself, many vaccine providers in the state have contracted with outside entities, such as Commonwealth Medicine, to assist with insurance billing for the costs of administering the vaccine. The Department is not a party to these agreements, but if your site has such an agreement you may wish to familiarize yourself with its terms.
Is there a cost to vaccine recipients?
Providers must administer COVID-19 Vaccine regardless of the vaccine recipient’s ability to pay administration fees or the recipient’s insurance coverage status. Providers may seek appropriate reimbursement from a program or plan that covers COVID-19 Vaccine administration fees for the vaccine recipient. Providers may not seek co-payment, reimbursement or any form of cost sharing, including through balance billing, from the vaccine recipient.
Is written consent needed for COVID-19 vaccination?
Informed consent is a vital process prior to the administration of a vaccine. DPH does not require a written informed consent form from vaccine recipients. However, vaccine providers should consult with their legal counsel regarding an appropriate informed consent process and what documentation may be recommended or required by their particular organization.
In order to make a vaccine clinic appointment in PrepMod, the individual making the appointment will be prompted to confirm the following:
The information I provided is correct.
I have been provided the COVID-19 EUA Fact Sheet for Recipients and Caregivers which has information about the risks and benefits of the vaccine. I will be able to ask questions at the time I receive my immunization.
I have the legal authority to and give consent for me and any other person(s) I registered to be vaccinated with the vaccine(s) above.
I give permission for my insurance company to be billed for the costs of administering the vaccine(s). The government is paying for the vaccine itself and I will not be billed for that portion of the cost of my immunization.
I understand that, as required by state law, all immunizations will be reported to the Department of Public Health Massachusetts Immunization Information System (MIIS). I can access the MIIS factsheet for Parents and Patients, at www.mass.gov/dph/miis, for information on the MIIS and what to do if I object to my or my family's data being shared with other providers in the MIIS.
What information do we need to provide about the US FDA Emergency Use Authorization (EUA) of the Moderna and Pfizer-BioNTech COVID-19 Vaccines?
The Department plans to make Moderna’s COVID-19 vaccine available to sites holding first responder vaccination clinics. Appropriate storage of and maintenance of cold chain for the vaccine will be necessary, and the Fact Sheet for Recipients and Care Givers must be made available to each individual receiving the vaccine.
The Moderna COVID-19 Vaccine Letter of Authorization (Letter) which describes the terms of the EUA, Fact Sheet for Healthcare Providers Administering Vaccine (provider fact sheet), and Fact Sheet for Recipients and Care Givers (recipient fact sheet) in addition to other related documents and translations of the fact sheet are available here: Moderna COVID-19 Vaccine | FDA. It is important to review the documents from the linked FDA site so that you have access to any updates or amendments.
Facilities, organizations, and healthcare providers holding vaccination clinics, as vaccination providers, should carefully review the Letter of Authorization and the provider and recipient fact sheets for the particular vaccine they will be administering. The Letter places obligations on vaccination providers including administering the vaccine in accordance with the EUA, making the recipient fact sheets available to each individual receiving the vaccine, and reporting certain information to the Vaccine Adverse Event Reporting System (VAERS).
In the event that it becomes necessary to provide the Pfizer-BioNTech COVID-19 vaccine for first responder clinics, the corresponding Letter and fact sheets in addition to other related documents and translations of the fact sheet should be carefully reviewed and are available here: Pfizer-BioNTech COVID-19 Vaccine | FDA. This Letter also places obligations on vaccine providers.
Site Considerations
What capacity does our site need to safely carry out COVID-19 vaccination best practices?
Screen patients for COVID-19 symptoms before and during the visit. Screening questions can be found in PrepMod, or sites can find sample forms on the CDC website.
Maintain physical distance (at least 6 feet apart, where possible).
Limit and monitor facility points of entry and install barriers to limit physical contact with patients at triage.
Observe respiratory hygiene (surgical facemasks for staff and face coverings for patients over 2 years of age, if tolerated) and cough etiquette.
Observe hand hygiene (including providing at least 60% alcohol hand sanitizer for patients).
Monitor individuals for possible adverse reactions. CDC recommends that persons without contraindications to vaccination who receive an mRNA COVID-19 vaccine be observed after vaccination for the following time periods:
30 minutes: Persons with a history of an immediate allergic reaction of any severity to a vaccine or injectable therapy and persons with a history of anaphylaxis due to any cause.
15 minutes: All other persons.
Perform enhanced surface decontamination. Detailed guidance for cleaning surfaces can found at this CDC site: https://www.cdc.gov/coronavirus/2019-ncov/community/disinfecting-building-facility.html
Refer to CDC guidance to prevent the spread of COVID-19 in health care settings, including outpatient and ambulatory care settings.
Ensure there is an adequate location to safely store the vaccine. Detailed information on safe storage and handling guidelines can be found here.
What type of refrigeration will we need?
LBOHs will be provided Moderna vaccine which can be stored at -15 to -25C for 6 months and at 2-8C for 30 days. DPH strongly encourages sites to store their Moderna vaccine frozen. Pharmaceutical and purpose-built refrigerators are a vaccine storage and handling best practice but are not required for the storage of COVID-19 vaccine. Standalone freezers are strong recommended, as the freezer portion of a household combination unit does not reliably maintain temperatures. If that is not possible for your site, please contact the Vaccine Unit at DPH-Vaccine-Management@massmail.state.ma.us for further guidance to ensure maintenance of appropriate temperatures. All storage units must be monitored continuously. The best practice for monitoring temperatures is to use a digital data logger available from DPH.
Operational Considerations
How should we set up our clinic?
Your current Emergency Dispensing Site (EDS) plan should serve as the foundation for standing up your first responder clinics. Review your plan to determine whether adjustments are necessary to support clinic operations and to provide for safe clinic set-up and flow. You can find the MDPH resource Emergency Dispensing Site (EDS): A Guide for Local Health On Planning for Medical Countermeasure (MCM) Dispensing Operations as well as other resources here.
If you are planning to set up a drive through clinic, please follow CDC guidance: Considerations for Planning Curbside/Drive-Through Vaccination Clinics | CDC. Please also note that the Interim Clinical Considerations for Use of mRNA COVID-19 Vaccine | CDC also state that for people with a history of any immediate allergic reaction to any other vaccine or injectable therapy, there should be an ability of the person to be vaccinated in a setting where appropriate medical care is immediately available for anaphylaxis.
How do sites that are holding first responder COVID-19 vaccination clinics order vaccine?
In Phase 1 of the Commonwealth’s COVID-19 Vaccine Plan, vaccine allocations will be determined by the MCVP; sites will not place orders. DPH will allocate vaccine to the site based on the availability of vaccine allocated by the federal government to Massachusetts, the information that the site provided in the MCVP Agreement, and information about the number of appointments scheduled. Once sites are able to place orders for vaccine directly, ordering will be done in the MIIS.
How do sites ask for more COVID-19 vaccine? (Updated 1/13/21)
On a weekly basis, all provider sites will receive a link to an MCVP survey. Completing this survey will ensure that DPH understands providers’ vaccine needs, the phase they are currently vaccinating in, as well as other information. Additionally, there is an option for the provider to also request additional doses as part of this survey process. If you have not received the weekly survey please email DPH-Vaccine-Management@massmail.state.ma.us.
How will sites that are holding first responder COVID-19 vaccination clinics receive the vaccine?
The Moderna COVID-19 vaccine will be delivered by UPS or FedEx to the sites you have identified to the MCVP. If working in a multi-community group, you may choose to have vaccine delivered to a single community and re-distributed to other community sites that meet all requirements for receipt of COVID-19 vaccine. Any community that will be receiving the vaccine either directly from UPS or FedEx or re-distributed through another community must be registered in the MIIS and have completed an MCVP Provider Agreement. In the MCVP Provider Agreement, ensure that you enter an accurate shipping address and shipping hours so that staff are on site to receive the vaccine shipment. We cannot guarantee that you will receive a phone call from the delivery driver when the shipment arrives.
To update your shipping address or hours, contact the Vaccine Unit at DPH-Vaccine-Management@massmail.state.ma.us Include your PIN and the contact email of who will update the Agreement. We will send out a link that will allow you to update the Agreement.
Can we take vaccine to EMS/police/fire stations rather than have them come to a clinic?
Yes, so long as you comply with all applicable requirements, including those for safe transport of vaccine. Consider the following general principles, which can also be found in the DPH COVID-19 Vaccine Training: The Moderna Supplement (PDF Slides).
Once a vial of vaccine has thawed, it may be stored refrigerated at 2° to 8° C for up to 30 days.
Once thawed, the vaccine cannot be re-frozen.
When thawed, the vaccine should be handled with care and protected from shocks, drops, vibration, etc.
Vaccine being transported at temperatures others than frozen (-15° to -25° C) should begin with the vaccine in the frozen state if at all possible.
If you must transport vaccine that has already been thawed, follow these general principles:
Punctured vials should not be transported.
Care must be taken to ensure vaccine does not re-freeze during transport.
Vaccine must be protected as much as possible from drops, shocks, and vibration whether in the carton, vial, case or cooler.
Vaccine should be transported in the carton whenever possible.
If transport must be conducted at the vial level, place the vial with dunnage (padding material like bubble wrap or similar padding) to minimize movement during transport.
The vaccine should always be transported in insulated containers qualified to maintain 2° to 8° C for the duration of transport.
The transport containers must be secured when being transported to prevent unnecessary movement.
After completion of transport, vaccine should immediately be placed into a vaccine storage unit at 2° to 8° C.
Vaccine should only be transported one time and should not be transported back again to the point of origin or to a new location.
Allowable timelines for transport of thawed vaccine are shown below. Total transport time should not exceed 12 hours in total.
Transport while walking or using hand cart: not to exceed 1 hour
Vehicle transport: not to exceed 12 hours
Airplane transport (rotary wing aircraft not permitted): not to exceed 3 hours
Will we need a standing order for the first responder clinics?
You will need to obtain a standing order for your program from a medical professional, such as a physician.
State law, M.G.L. c. 94C, section 8 (7), requires a licensed provider with prescribing authority to issue an order for administration of a vaccine such as the COVID-19 vaccine.
Authorized ordering providers include, a: physician, chiropractor, surgeon, podiatrist, osteopath, nurse practitioner, dentist, or physician’s assistant. See MGL Ch. 94C; 105 CMR 700.00.
A standing order is an order issued by a licensed provider, which is not specific to one person, and enables assessment and vaccination of patients without the need for clinician examination or direct order from the attending provider at the time of the interaction.
The standing order should be specific about which clinics and what dates or periods of time are covered, e.g., “COVID-19 vaccination clinics for first responders operated by (name of organization, LBOH, coalition, etc.) from January 4, 2021 through February 28, 2021.”
Any individual who meets the criteria included in a standing order may receive the vaccine consistent with the terms of the order.
A model standing order developed by CDC for the Moderna COVID-19 vaccine can be found here.
Other Emergency Treatment Standing order templates are available from the Immunization Action Coalition:
Medical Management of Vaccine Reactions of Adults in a Community Setting
Medical Management of Vaccine Reactions in Children and Teens in a Community Setting
How do we notify and schedule first responders for our clinic?
Appointments are strongly encouraged to ensure that there is no wasted vaccine and to help manage queuing and social distancing. If a site chooses to allow walk-ins, there is no guarantee that they will be able to be vaccinated.
If you have a system you currently use to schedule appointments, you can continue to use that. You must be able to generate a 2nd dose reminder for vaccine recipients through your electronic scheduling system or by written reminder. You will also need to manually enter the mandated data into the MIIS within the federally required 72 hours after administering vaccine. As an alternative, DPH will be offering PrepMod, which can be used to schedule, screen, bill, and report to the MIIS. PrepMod will be available through a link on the COVID-19 webpage, and first responders will be able to register for available clinics. If you use PrepMod, your clinic information and scheduling availability will be listed on the website. More information will be shared about PrepMod soon.
Do we need to have 200 confirmed appointments to receive vaccine?
You must have identified at least 200 first responders eligible for vaccination and have the capacity to vaccinate at least 200 first responders, but you do not have to have 200 confirmed appointments to receive vaccine.
What should a site do with extra vaccine (e.g. if we get more doses than we have first responders who are willing to be vaccinated)? (Updated 1/6/21)
Sites can continue to vaccinate anyone in a currently eligible priority group in accordance with the Commonwealth’s COVID-19 Vaccine Plan, so COVID-facing health care workers in the community (including vaccinators and COVID testers) can also be vaccinated with excess vaccine because both COVID-facing health care workers and first responders will be in currently eligible priority groups. Vaccine can also be transferred to small primary care practices, that meet all requirements for receipt of COVID-19 vaccine, for their eligible staff. Such a transfer must be done in consultation with the Vaccine Unit. Visit Massachusetts COVID-19 Vaccine Program (MCVP) – Guidance for Healthcare Providers and Organizations | Mass.gov for more guidance on redistribution.
Where will people receive their second dose?
The expectation is that the people will receive their second dose from the same clinic or other clinics in the same area. Alternatively, people can get their second dose at a mass vaccination clinic site or primary care provider. It is important for people to receive the second dose to ensure full efficacy. For COVID-19 vaccines requiring 2 doses, the 2nd dose must be the same vaccine product as the first dose.
How will people know which vaccine product they receive and when they need the second dose?
The vaccine ancillary supply kits will come with vaccine record cards that can be given to the recipients indicating what vaccine they received and that they need a second dose. Vaccine record cards may be reproduced, if necessary. In addition, there are electronic reminder/recall systems in the MIIS that providers could use in addition to their own EHR systems to send reminders to recipients about their second dose. More information about the v-safe app, which also includes a reminder recall, can be found at V-safe After Vaccination Health Checker | CDC. Providers should schedule the 2nd dose at the time the 1st dose is administered.
What vaccine and ancillary supplies will sites that are holding first responder COVID-19 vaccination clinics receive?
The first responder clinics will receive the Moderna COVID-19 vaccine. Hospitals that are working with local health to vaccinate their first responders will receive Pfizer. The Standard COVID-19 Vaccine Adult Ancillary Kit supports administration of 100 doses and includes needles, syringes, alcohol pads, vaccination record cards, needle guide, face shields, and face masks. Additional details about these supplies can be found at Massachusetts COVID-19 Vaccine Program (MCVP) – Guidance for Healthcare Providers and Organizations | Mass.gov.
Are we responsible for printing and making available information on v-safe? (Updated 1/12/21)
Yes. V-safe is a new voluntary, smartphone-based tool that uses text messaging and web surveys to provide personalized health check-ins for COVID-19 vaccine recipients. V-safe allows people to report any side effects after COVID-19 vaccination to CDC in almost real time. It also gives them a convenient reminder to get their second COVID-19 vaccine dose if they need one. Provide the v-safe Information Sheet or QR code to every vaccine recipient and encourage them to enroll and complete the surveys when prompted to do so. For more information, or to register for v-safe, visit: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html
Staffing Considerations
Who can administer vaccine? (Updated 1/13/21)
This COVID-19 Vaccinators chart lists the categories of health professionals who can possess and administer COVID-19 vaccines. EMTs and paramedics supporting vaccine clinics must be employed by a licensed ambulance service and be trained and operating with the approval of their Affiliate Hospital Medical Director. Please review the Office of Emergency Medical Services advisories and protocols.
What qualifications do individuals need to operate the program and administer the vaccine?
All individuals who receive vaccine deliveries, handle, or administer vaccines must be trained in vaccine related practices and procedures. They should be able to ensure the safety and efficacy of vaccines through proper:
Benefit and risk communication
Vaccine storage/handling and administration
Timing and spacing of vaccine doses
Screening for contraindications and precautions
Management of adverse reactions
Being able to access and use emergency equipment
Current CPR certification
Reporting to VAERS (and any additional COVID specific databases)
Documentation
If you will be using volunteer vaccinators for your clinics, make sure that everyone is up-to-date with their vaccinating skills. You can use the checklist below and, if needed, have the volunteers watch the training video.
Do sites that are holding first responder COVID-19 vaccination clinics always have to have one Vaccine Coordinator and one Backup Vaccine Coordinator on site for the duration of a clinic?
Yes, at least one of these individuals should be on site at all times.
Is there a training for vaccinators?
The primary and back-up vaccine coordinators at each site and providers administering COVID-19 vaccine are encouraged to complete the Introduction to COVID-19 Vaccine Storage & Handling and Administration Training, which has a focus on the Pfizer-BioNTech COVID-19 vaccine. Recording and slides from the training are below:
COVID-19 Storage and Handling Training PDF Slides | (Accessible)
COVID-19 Vaccine Administration Training PDF Slides | (Accessible)
Vaccine coordinators and providers are also strongly encouraged to complete the COVID-19 Vaccine Training: The Moderna Supplement. This training is designed as supplemental training to the COVID-19 Vaccine Storage & Handling and Administration training listed above. It includes updates and is designed for health care providers, vaccine coordinators, and all health care personnel who handle and/or administer vaccines. Recording and slides from the training are below:
What personal protective equipment (PPE) is needed?
Each site must provide its own PPE for clinic sites and ensure an adequate supply including:
Surgical Masks
Required: All health care providers (N95 masks not recommended)
Eye protection
Required: Areas of moderate/substantial community transmission or if ultra-cold/dry ice is being handled
Optional: Areas of minimal/no community transmission
Gloves
Required: Latex or similar gloves needed to administer intramuscular or subcutaneous vaccine
Required: If ultra-cold or dry ice are being handled, special insulating gloves are needed
Clinical Considerations
For both the Pfizer and Moderna vaccines, there may be enough extra in each vial for more than the standard 5 or 10 doses. Is it okay to administer additional doses from a vial? (New 1/7/21)
Yes. After preparation, vials of Pfizer-BioNTech COVID-19 Vaccine contain up to six doses of 0.3 mL. Low dead-volume syringes and/or needles can be used to extract up to six doses from a single vial. If standard syringes and needles are used, there may not be sufficient volume to extract a sixth dose from a single vial. Irrespective of the type of syringe and needle:
Each dose must contain 0.3 mL of vaccine.
If the amount of vaccine remaining in the vial cannot provide a full dose of 0.3 mL, discard the vial and any excess volume.
Do not pool excess vaccine from multiple vials.
As a reminder, use only the prescribed 1.8 ml of diluent when reconstituting a vial.
Vaccinators may also find that they can withdraw more than 10 doses of the Moderna COVID-19 vaccine from a single 10-dose vial.
Extra vaccine fluid from more than one vial CANNOT be combined to produce extra doses.
Use any extra vaccine that can easily be drawn into a syringe from one vial to meet the 0.5 ml dose requirement.
Enter all vaccine doses administered into the MIIS.
What should sites that are holding first responder COVID-19 vaccination clinics do with unused doses in the Moderna COVID-19 vaccine multi-dose vial?
Once a vial of Moderna COVID-19 vaccine has been entered, it must be used within 6 hours. Be sure to note the time the vial was first entered on the vial. Any vaccine remaining in the vial after 6 hours must be discarded.
Careful planning is important to ensure that COVID-19 vaccine is not wasted. It is essential that you have 10 people confirmed for vaccination within 6 hours before entering a new vial for the first. In the rare instance where you have COVID-19 vaccine that will expire and you have no one in the current priority groups to be vaccinated, you can use your clinical judgement to administer the vaccine to a person in another priority group who is closest to the current priority group being targeted for vaccination to avoid vaccine waste. It is important that you also ensure that this individual is now included in your reminder recall systems for the 2nd dose.
What are the potential side effects of the vaccine?
Systemic signs and symptoms, such as fever, fatigue, headache, chills, myalgia, and arthralgia, can occur following COVID-19 vaccination. Preliminary data from mRNA COVID-19 vaccine trials indicate that most systemic post-vaccination signs and symptoms are mild to moderate in severity, occur within the first three days of vaccination (the day of vaccination and following two days, with most occurring the day after vaccination), resolve within 1-2 days of onset, and are more frequent and severe following the second dose and among younger persons compared to those who are older (>55 years). Cough, shortness of breath, rhinorrhea, sore throat, or loss of taste or smell are not consistent with post-vaccination symptoms, and instead may be symptoms of SARS-CoV-2 or another infection.
Should someone who is COVID-positive receive the vaccine?
Vaccination of persons with known current SARS-CoV-2 infection should be deferred until the person has recovered from the acute illness (if the person had symptoms) and criteria have been met for them to discontinue isolation. While there is otherwise no recommended minimum interval between infection and vaccination, current evidence suggests that reinfection is uncommon in the 90 days after initial infection. Thus, persons with documented acute SARS-CoV-2 infection in the preceding 90 days may delay vaccination until near the end of this period, if desired.
Should people who have had COVID-19 be vaccinated?
Yes, people who have previously had COVID-19 should be vaccinated. Though it is uncommon to be re-infected in the 90 days after initial infection, people may choose to delay vaccination until the end of this period.
Can the vaccine be given to people who are pregnant?
There are no data on the safety of COVID-19 vaccines in people who are pregnant. Animal developmental and reproductive toxicity (DART) studies are ongoing. Studies in humans are also ongoing and more are planned.
If a person is part of a group (e.g., healthcare personnel) who is recommended to receive a COVID-19 vaccine and is pregnant, that person may choose to be vaccinated and may wish to discuss with their healthcare provider.
mRNA vaccines are not live vaccines. They are degraded quickly by normal cellular processes and don’t enter the nucleus of the cell.
COVID-19 infection during pregnancy can result in an increased risk of severe illness (ICU admission, mechanical ventilation and death) and might result in an increased risk of adverse pregnancy outcomes, such as preterm birth.
Consider the following when discussing COVID-19 vaccination with people who are pregnant:
Level of COVID-19 community transmission, (risk of acquisition)
Personal risk of contracting COVID-19, (by occupation or other activities)
The risks of COVID-19 to the person who is pregnant and potential risks to the fetus
The efficacy of the vaccine
The known side effects of the vaccine
The lack of data about the vaccine during pregnancy
Pregnant people who experience fever following vaccination should be counseled to take acetaminophen as fever has been associated with adverse pregnancy outcomes.
Routine testing for pregnancy prior to receipt of a COVID-19 vaccine is not recommended.
Update #95: Vaccination Plans for First Responders (911 Dispatchers Added), Vaccine Phase 2 Priority Group Adjustment, Daily Vaccination Dashboard
Today, the Baker-Polito Administration outlined plans to vaccinate the Commonwealth’s first responders, the next priority group within Phase One of the Commonwealth’s COVID-19 vaccine distribution plan. The Administration also launched a new interactive COVID-19 daily dashboard to provide a more user-friendly tool for the public to access data on the impact of COVID-19 in Massachusetts.
Baker-Polito Administration Outlines Vaccination Plans for First Responders, Provide Vaccine Update
· January 4, 2021 Press Conference (video)
· COVID-19 Vaccine Locations for First Responders (Please note: 911 Dispatchers have been added to the first responder list.)
· Vaccine Phase 2 Priority Group Adjustment: The Commonwealth updated its Vaccine Distribution plan so that individuals age 75+ are included in Phase 2, Group 1, along with individuals of all ages with 2+ comorbidities
· When Can I Get the COVID-19 Vaccine (Please note: The webpage is updated frequently; please check back often.)
· COVID-19 Daily Vaccination Dashboard
· COVID-19 Vaccine e-mail address COVID-19-Vaccine-Plan-MA@mass.gov
Thank you for all you have done and continue to do to keep people safe and save lives.
Two Updates from DPH/OEMS
New PPE Preservation Planning Toolkit Now Available
The HHS Office of the Assistant Secretary for Preparedness and Response (ASPR) has developed a new toolkit, including links to a guide and an Excel spreadsheet, to assist organizations with planning and implementation strategies for personal protective equipment (PPE) preservation. Developed by ASPR’s COVID-19 Healthcare Resilience Working Group, the toolkit can help users understand types of PPE preservation strategies and calculate how using those strategies can increase the duration of a specified PPE supply.
MDPH COVID-19 PPE Stockpile Survey
Massachusetts Department of Public Health (MDPH) in collaboration with Massachusetts General Hospital Center for Disaster Medicine (MGH) and the Massachusetts Institute of Technology Humanitarian Supply Chain Lab (MIT) have developed the MDPH COVID-10 PPE Stockpile Survey, which is intended to inform the development of a state-level cache of PPE designed to support the emergent/urgent needs of Massachusetts healthcare organizations. Robust participation by healthcare organizations within Massachusetts will enable the development of a plan that can best meet the diverse needs of our collective organizations. Therefore, we would like to ask that you please take a moment to complete the survey for your organization.
Below are the link to the survey as well as a QR code to the survey.
Survey link https://www.surveymonkey.com/r/G53GPWM
Questions or feedback can be directed to Roberta Roberta.Crawford@Mass.gov
Safe Ventilation Use Reminder
The Massachusetts Department of Public Health (DPH) continues to work with state, federal and local partners on the outbreak of novel Coronavirus 2019 (COVID-19), caused by the virus SARS-CoV-2, and we continue to appreciate the essential role you have in responding to this evolving situation.
The purpose of this email is to remind ambulance services that in accordance with the Statewide Treatment Protocols’ emergency protocol 1.2 Patient at Risk of COVID-19, https://www.mass.gov/doc/emergency-release-of-12-patient-at-risk-for-coronavirus-disease-2019-covid-19-updated-april-0/download, it is critical that EMS take appropriate precautions when treating a patient who needs ventilation. When using a ventilator or bag-valve mask on a patient, EMS is to ensure the ventilation device has an in-line HEPA filter, whether the patient is intubated or not. This is essential to prevent potential COVID-19 exposure of the EMS and receiving hospital personnel.
Dear EMS Colleagues,
The Massachusetts Department of Public Health (DPH) continues to work with state, federal and local partners on the outbreak of novel Coronavirus 2019 (COVID-19), caused by the virus SARS-CoV-2, and we continue to appreciate the essential role you have in responding to this evolving situation.
The purpose of this email is to remind ambulance services that in accordance with the Statewide Treatment Protocols’ emergency protocol 1.2 Patient at Risk of COVID-19, https://www.mass.gov/doc/emergency-release-of-12-patient-at-risk-for-coronavirus-disease-2019-covid-19-updated-april-0/download, it is critical that EMS take appropriate precautions when treating a patient who needs ventilation. When using a ventilator or bag-valve mask on a patient, EMS is to ensure the ventilation device has an in-line HEPA filter, whether the patient is intubated or not. This is essential to prevent potential COVID-19 exposure of the EMS and receiving hospital personnel.
EMS personnel are reminded that Heat and Moisture Exchanger Filters (which may be attached to circuits during IFT) are not High Efficiency Particulate Air (HEPA) filters and are not adequate when used alone.
If in-line HEPA filters are not available, ventilation must be considered an aerosolizing procedure, and the EMS personnel should wear fitted N95 or equivalent respirators. EMS must also notify receiving facilities that an incoming patient is being ventilated without an in-line HEPA filter, so hospital personnel can don appropriate personal protective equipment (PPE).
Continuous positive airway pressure (CPAP) and bi-level positive airway pressure (BiPAP) are inherently aerosolizing, and while a HEPA filter on the circuit does not provide sufficient protection to forego N95 or equivalent respirators, EMS personnel should to include an in-line HEPA filter if one is available. In general, it is best to defer CPAP or BiPAP use to the hospital setting where a patient can be in a filtered room, in accordance with emergency protocol 1.2.
If you have any questions, please contact Brendan Murphy at Brendan.P.Murphy@mass.gov.
The DPH COVID-19 website, which is updated on a daily basis, can be accessed at www.mass.gov/2019coronavirus.
Thank you,
Scott
W. Scott Cluett III, NRP
Director
Office of Emergency Medical Services
Bureau of Health Care Safety and Quality
Massachusetts Department of Public Health
67 Forest Street
Marlborough MA 01752
Office - 617-753-8110 – Pls use mobile. Working remotely for COVID19
Mobile - 857-348-3163
EMS Recruitment for COVID-19 Vaccine Trial
Brigham and Women’s Hospital researchers are looking for volunteers to participate in a COVID-19 Vaccine research study. Attached is some informational material regarding this Trial. If you are interested or if you have any questions, please contact a member of their study staff by Phone: 978-822-2463 or E-mail: vaccines@partners.org. The timeline is tight, as there are limited spots and enrollment has already begun.
For more information, visit their website bit.ly/covidvax or in Spanish bit.ly/covidvax-esp.
● Their team is recruiting folks in the Boston area for this study. However, they are part of a national network (the Coronavirus Prevention Network, or CoVPN) which is recruiting throughout the country.
● They are broadly recruiting folks who are at increased risk of COVID-19. Their main exclusion criteria are people who have had COVID-19 and people who are pregnant.
● The study consists of around seven (7) scheduled visits over the course of two (2) years. The study involves a receiving vaccine or placebo, blood draws, and health monitoring. Their team is conducting this study at three (3) locations in Boston:
Brigham and Women's Hospital
75 Francis Street, Boston, MA 02115
Southern Jamaica Plain Health Center
640 Centre St, Jamaica Plain, MA 02130
Brigham and Women’s Health Care Center
830 Boylston Street, Chestnut Hill, MA 02467
● Interested participants in the Boston area can go to their Rally page (bit.ly/covidvax) which has more information about this study, and contains their pre-screening questionnaire. You can also learn more about their study team and research at bwhvaxx.org. Paulette Chandler, MD, MPH, Assistant Professor of Medicine, Harvard Medical School; Associate Epidemiologist, Division of Preventive Medicine; Associate Physician, Phyllis Jen Center of Primary Care at Brigham and Women’s Hospital is willing to discuss the study with you by phone. Dr. Chandler’s phone numbers are clinic 617-732-6043, office 617-732-8574 or email pchandler@bwh.harvard.edu .
UPDATED Comprehensive Personal Protective Equipment (PPE) Guidance
The Massachusetts Department of Public Health continues to work with state, federal and local partners on the outbreak of Coronavirus Disease 2019 (COVID-19), caused by the virus SARS-CoV-2, and we continue to appreciate the essential role you have in responding to this evolving situation.
The Department of Public Health has developed updated comprehensive PPE guidance (see attached), based upon changes made to the Centers for Disease Control and Prevention recommendations about eye protection.
Comprehensive Personal Protective Equipment (PPE) Guidance
The Department of Public Health has released updated Comprehensive Personal Protective Equipment (PPE) Guidance for healthcare facilities. This updated guidance is also posted on the DPH’s COVID-19 website: Personal Protective Equipment (PPE) During COVID-19: Guidance.
Deadline for Battelle Critical Care Decontamination System N95 Masks THIS SUNDAY
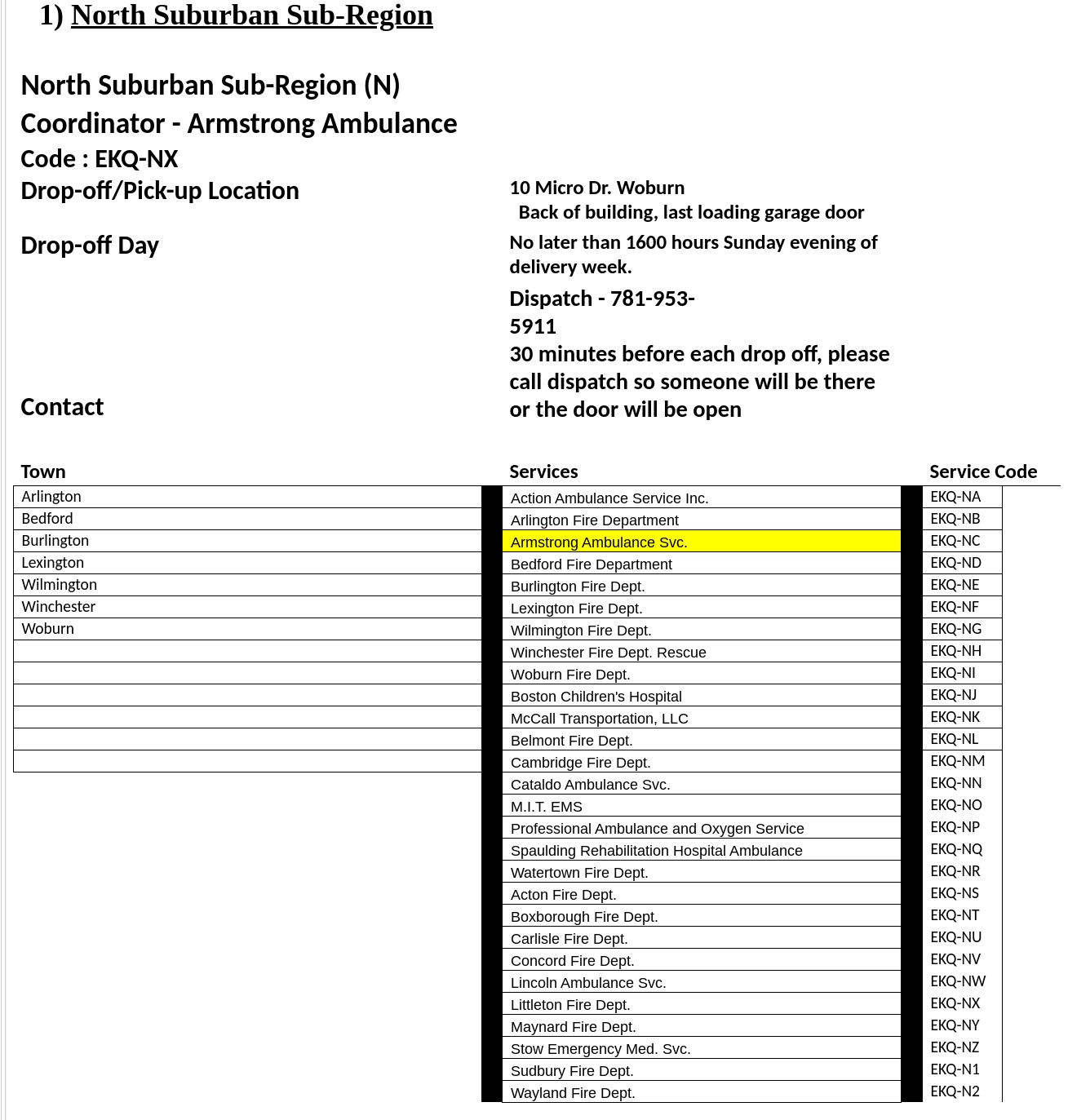
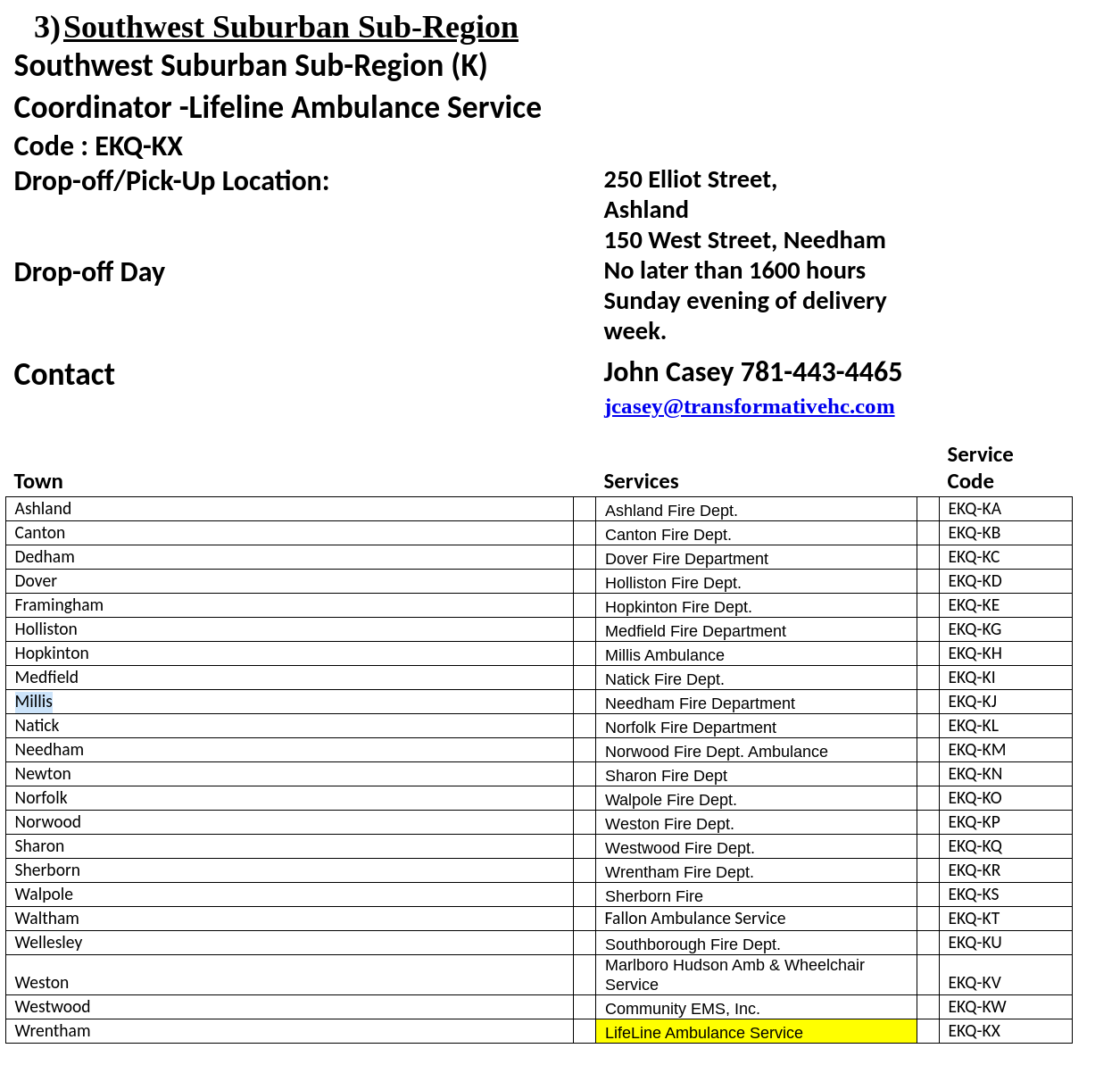
The collection period the deadline for this collection is Sunday May 3rd, no later than 1600 hours, with the exception of the South Suburban Sub-Region as indicated on their information below.. You will need to contact the sub-region designated point of contact prior to delivery to ensure that someone will be there to receive your delivery. Please see the following Region IV Sub-region designation form for points of contact, delivery locations, and service identification codes.
Should you have questions regarding any of the material, please contact Derrick Congdon, Executive Director, via email at dcongdon@mbemsc.org.
Instructions for Region IV EMS Services and Sub-region Coordinators: Preparation and Collection of Compatible N95 Respirators for Decontamination by the Battelle Memorial Institute Using the Battelle Decontamination System
The U.S. Food and Drug Administration has authorized an Emergency Use Authorization (EUA) for the emergency use of the Battelle CCDS Critical Care Decontamination System™ (hereafter referred to as the “Battelle Decontamination System”) operated by the Battelle Memorial Institute (“Battelle”), for use in decontaminating compatible N95 or N95-equivalent respirators (“compatible N95 respirators”), for reuse by healthcare personnel. Healthcare personnel should follow these instructions, as well as procedures at their healthcare facility, to prepare compatible N95 respirators for decontamination by Battelle using the Battelle Decontamination System.
• Due to incompatibility, the Battelle Decontamination System is not authorized for use with respirators containing cellulose-based materials
• All compatible N95 respirators provided to Battelle must be free of any visual soiling or contamination (e.g., blood, bodily fluids, makeup)
• If N95 respirators are soiled or damaged, they will be disposed of and not returned after decontamination
INSTRUCTIONS
EMS SERVICE AND EMTs
On-Site Collection/Marking
1. EMS services should create a collection station at the point of generation (i.e. garage/ambulance base)
2. Each station should have a bag provided by the service to collect compatible N95 respirators. NOTE: Bags are for compatible N95 respirators only. Do not throw other personal protective equipment (such as gloves), paper towels, or waste in the collection bag.
3. With a permanent marker, the EMS staff should label their own individual compatible N95 respirators with a three-digit site code and a 2-digit location identifier (as shown below). The unique three (3) digit site code corresponds to the Metropolitan Boston EMS Council (Region IV). The two (2) digit location identifier corresponds to a specific sub-region with in Region IV and the individual EMS service.
4. EMS staff should follow the instructions provided by Battelle in Instructions for Healthcare Personnel: Preparation of Compatible N95 Respirators for Decontamination by the Battelle Memorial Institute Using the Battelle Decontamination. (See Attached Instructions for Healthcare Personnel)
Region Code Sub-region/Service Code
EKQ - SA
Preparation for Shipment by EMS Service:
1. Bags containing the contaminated compatible N95 respirators to be decontaminated by Battelle (“primary collection bag” provided by the EMS service) should be closed.
2. Place the primary collection bag into another bag (“secondary collection bag” provided by the EMS service), which is then closed.
3. Decontaminate the secondary collection bag with alcohol or other suitable decontaminant.
4. Place the decontaminated bags into a rigid, closed box (i.e. cardboard), supplied by the EMS service, clearly labeled with a biohazard symbol, and tape the box securely shut.
5. Label the outside of the box with the 3-digit region code and 2-digit location identifier.
6. Complete a Region IV Chain of Custody Form for your EMS Service.
7. Deliver the completed box to the designated Region IV sub-region coordinator for transport to Battelle. All deliveries to the sub-region coordinators must be completed by 16:00 hours, the Sunday evening before transfer date to Battelle beginning April 26, 2020. This will be the first delivery to Battelle. Once we determine the number of PPE processed during this shipment, we will determine if collections will be weekly or bi-weekly moving forward.
SUB-REGION COORDINATOR
Sub-region Shipment of completed boxes is under the Metropolitan Boston EMS Council agreement with Battelle:
1. Gather all boxes at designated sub-region location (see attached spreadsheet for your exact Sub-region location); completed one chain of custody form (provided by Battelle) per shipment, noting the number of boxes.
2. Transfer boxes to designated Battelle location in Somerville, Massachusetts for decontamination.
3. Transfer decontaminated PPE from Battelle location in Somerville, Massachusetts back to sub-region for redistribution.
4. Notify Region when decontaminated PPE has been returned from Battelle, so the services in the sub-region can be notified to pick-up at the designated location.
Reuse Information:
Following decontamination, you will be provided decontaminated compatible N95 respirators that have been processed through a decontamination system for reuse by healthcare personnel in a healthcare setting during the COVID-19 pandemic. Before reuse, the healthcare facility should review the chain of custody form, which indicates successful decontamination, accompanying the returned respirators. The healthcare facility should also inspect each returned, decontaminated compatible N95 respirator for:
1. Numeric indication of the decontamination cycle number. NOTE: Compatible N95 respirators will be disposed of after 20 decontamination cycles.
2. Visible damage or soiling. NOTE: Compatible N95 Respirators should be discarded and not reused if visually damaged or soiled.
Any problems should be immediately reported to Battelle and the Metropolitan Boston EMS Council.
Battelle Contact: 1-800-201-2011 or solutions@battelle.org
Metropolitan Boston EMS Council Contact: 781- 505-4367 or dcongdon@mbemsc.org
Guidance for EMS on the Use of PPE in Long Term Care Facilities
The purpose of this communication is to share new guidance on the use of Personal Protective Equipment (PPE) by First Responders and EMS when entering a nursing home or rest home (long term care facility).
In alignment with the Centers for Medicare and Medicaid Services (CMS) and due to the widespread community transmission of COVID-19, the Department of Public Health (DPH) is advising all first responders, including but not limited to, fire personnel, law enforcement, and EMS personnel, to assume when responding to an event at a long term care facility that the individuals they are interacting with are infected with COVID-19. First responders and EMS personnel should don the appropriate PPE recommended for caring for individuals who are infected with COVID-19.
There is also a document with Nursing Home Frequently Asked Questions (FAQs) for First Responders & Municipalities.
OEMS to issue Temporary EMT Certifications
The Massachusetts Department of Public Health (DPH) continues to work with state, federal and local partners on the outbreak of novel Coronavirus 2019 (COVID-19), caused by the virus SARS-CoV-2, and we continue to appreciate the essential role you have in responding to this evolving situation.
In accordance with the Commissioner of Public Health’s April 3, 2020 order maximizing health care provider availability, to respond to Governor Baker’s declared State of Emergency, the Department is issuing temporary EMT certification, upon request, to applicants who either:
Currently hold EMT certification/licensure, at any level) in another state, which has not been revoked, suspended, surrendered or are otherwise not in good standing in another state; OR
Previously held Massachusetts EMT certification, but it has expired within the last 10 years (at any level). This does not include certifications that have been revoked, suspended or surrendered.
Applicants may request these temporary certifications by emailing oems.recert@state.ma.us, or by completing the attached application form (soon to be available on the DPH/Office of Emergency Medical Services website), and submitting it to the office by FAX or mail, along with copies of their current CPR credential (and ACLS for Paramedics). DPH will not accept or review requests for temporary certification from applicants who do not have current CPR or ACLS, as appropriate to their level, credentials. Note that due to the COVID-19 State of Emergency, any CPR or ACLS credential that expired on or after February 1, 2020, is deemed current through July 1, 2020. Applicants who currently or have previously held certification in other states will need to provide a verification from the other states (the applicant must request verification from the state in which the applicant is certified; that state (or states) must complete the form and submit it directly to DPH/OEMS). National Registry of EMTs (NREMT) certification is not required for temporary certification, and there is no fee.
Once OEMS receives a complete application, we will expedite review and processing, and temporary EMTs will receive an email confirmation once the temporary certification has been issued. Temporary certifications will be immediately visible at https://checkalicense.hhs.state.ma.us, and may be verified there at any time.
Temporary certifications will remain valid until the termination of the state of emergency for out of state EMTs, and 90 days following the termination of the state of emergency for previously MA certified EMTs. Temporary EMTs who wish to become fully certified as EMTs after their temporary certification expires must meet all the standard regulatory entry/reinstatement requirements (including holding National Registry certification).
Temporary EMTs may function in connection with a licensed ambulance service at the full scope of practice for their level – but must do so in accordance with Statewide Treatment Protocols and must have received authorization to practice from the service’s affiliate hospital medical director. Ambulance services are responsible for ensuring Temporary EMTs receive required education/orientation and authorization to practice before they begin to work.
Please contact oems.recert@state.ma.us with any additional questions
OEMS Issues Comprehensive Personal Protective Equipment (PPE) Guidance
The purpose of this communication is to share comprehensive guidance, based upon the Centers for Disease Control and Prevention (CDC) recommendations, to clarify the Personal Protective Equipment (PPE) that health care personnel (HCP) use in the clinical care areas, particularly during this time when we are optimizing our supplies.
This guidance includes information about facemasks, eye protection, isolation gowns, gloves, respirators, and PPE for COVID-19 Patient Care.
The Massachusetts Department of Public Health (DPH) continues to work with state, federal and local partners on the outbreak of Coronavirus Disease 2019 (COVID-19), caused by the virus SARS-CoV-2, and we continue to appreciate the essential role you have in responding to this evolving situation.
Temporary MIH Applications for COVID-19
The Massachusetts Department of Public Health (DPH) continues to work with state, federal and local partners on the outbreak of novel Coronavirus 2019 (COVID-19), caused by the virus SARS-CoV-2, and we continue to appreciate the essential role you have in responding to this evolving situation.
In response to the COVID-19 outbreak, DPH is releasing the below document:
Memorandum providing guidance on applying for a temporary Mobile Integrated Health Care (MIH) license during the duration of the COVID-19 emergency. DPH will waive fees and expedite review for applicants that meet the requirements outlined in the guidance.
For any questions about the Protocol, please contact please contact the MIH manager at MIH@state.ma.us or via phone at (617) 753-8484.
The DPH COVID-19 website, which is updated on a daily basis, can be accessed at www.mass.gov/2019coronavirus.